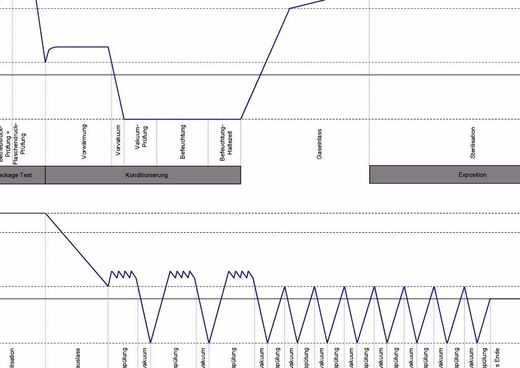
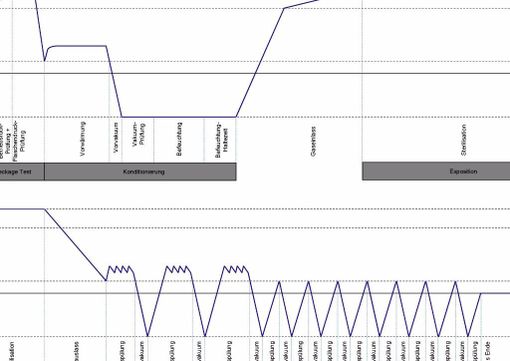
Our SteriVIT Process
Ethylene oxide gas penetrates material even at lowest temperatures and inactivates bacteria, viruses and funghi. Therefore, it is the method of choice for the sterilization of thermo-labile products. By means of the overpressure method and by using low ethylene oxide concentrations, we are able to sterilize in a reliable, efficient and reproducible way even narrow-lumened products and geometries which are hard to penetrate. A low agent concentration ensures the integrity of your products equipped with drugs, whereas other sterilization methods reach their performance limits here. Conditioning, sterilization and desorption are done in a fully-automatic process and guarantee procedurally safe and easy handling. The processes required for your products are checked in the framework of a validation. Here, it is proved that all valid standards have been met with regard to microbiology and EtO residues. The data recorded during the sterilization allows a reliable parametric release of the sterilization process. Thus, no laboratory analytics are required, which saves time and money.
The SteriVIT process is synonymous with a modern, up-to-date sterilization of your medical devices. Latest findings in the field of process engineering support our future-oriented and environmentally friendly technology
Parameters of parametric release:
- Pressure
- Humidity
- Temperature
- Agent
- Time